40 fda relaxing food labels
Import Alert 66-41 - Food and Drug Administration If a firm and/or a representative thereof would like to petition for removal from detention without physical examination under this Import Alert, all relevant information supporting the request should be forwarded to the following address: Food and Drug Administration Division of Import Operations 12420 Parklawn Drive, ELEM-3109 Rockville, MD 20857 Or, be sent via email to: … Buy CBD Gummies & CBD Edibles Online | Green Roads The FDA forbids all CBD companies from publishing reviews in which customers mention specific health conditions. As you might imagine, a large number of our consumer reviews mention specific health conditions. In order to comply with the FDA, we’ve published a representative selection of reviews that pass the FDA guidelines. These are actual, unaltered reviews from …
Food label policy changes - The Mast Cell Disease Society, Inc In response to the Food and Drug Administration's (FDA) announcement last week, we joined 11 other organizations to issue a joint letter to the agency about its interim food labeling guidelines in response to supply chain issues and shortages brought on by the coronavirus pandemic.

Fda relaxing food labels
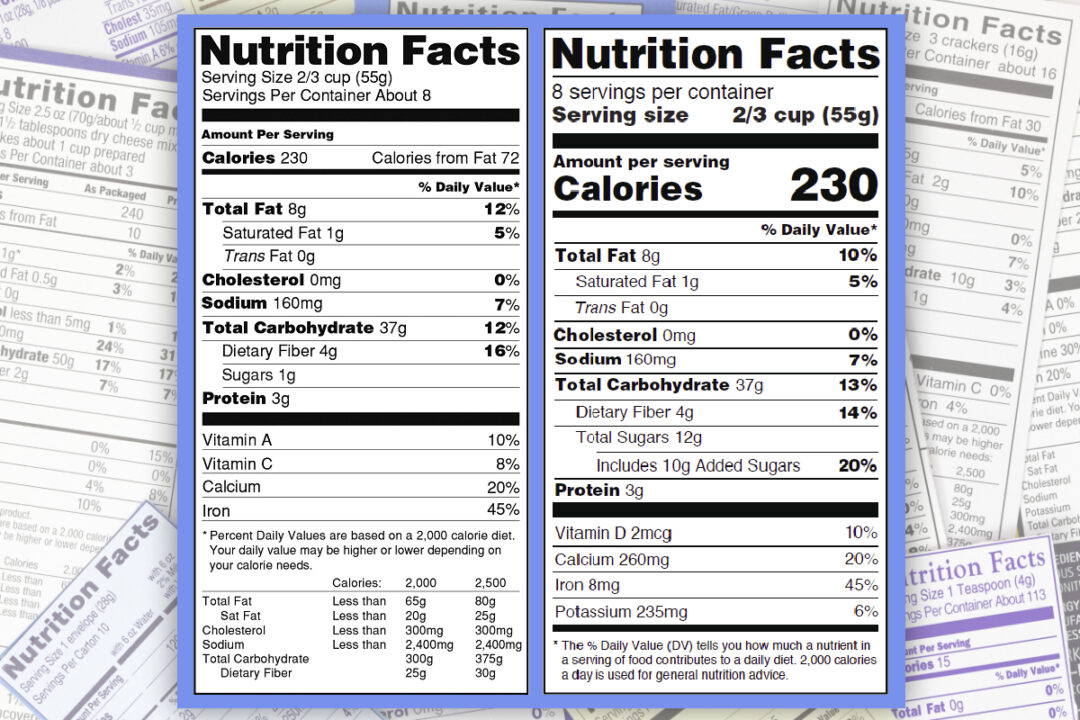
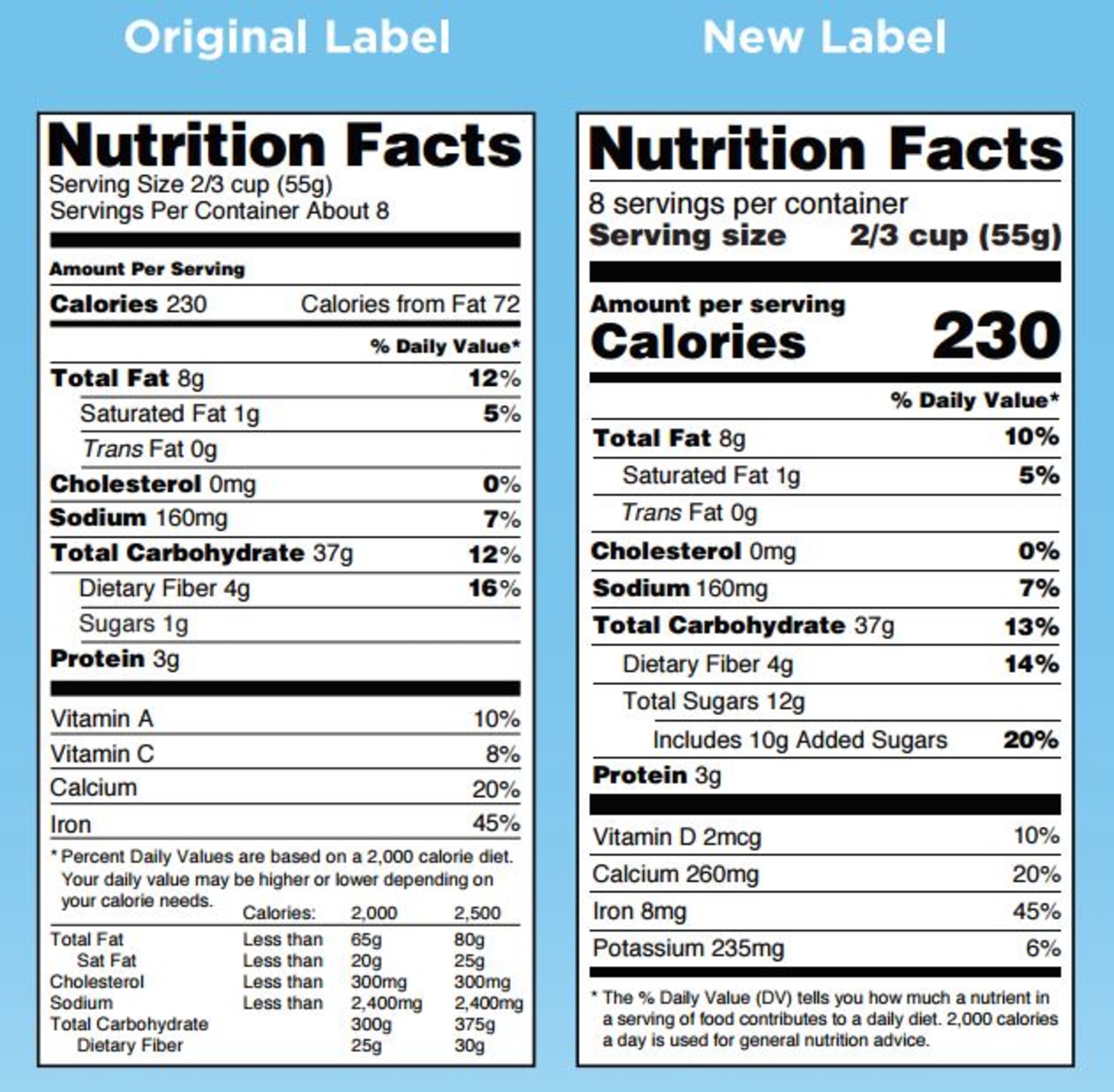
Food Allergen Labeling—What Happens Next? - Celiac.com However, Covid-19 has changed that. Responding to calls from food manufacturers facing supply chain problems in the wake of the Covid-19 pandemic, the Food and Drug Administration (FDA) is relaxing some labeling requirements for certain ingredients in food in the U.S. Amazon.com: Medisential Enema Kit - Suitable for Coffee, Water … Statements regarding dietary supplements have not been evaluated by the FDA and are not intended to diagnose, treat, cure, or prevent any disease or health condition. Statements regarding dietary supplements have not been evaluated by the FDA and are not intended to diagnose, treat, cure, or prevent any disease or health condition. Customer reviews. 4.6 out of 5 stars. 4.6 out … FDA Provides Temporary Flexibility Regarding Nutrition Labeling of ... FDA Provides Temporary Flexibility Regarding Nutrition Labeling of Certain Packaged Food in Response to the COVID-19 Pandemic Constituent Update March 26, 2020 As a result of the COVID-19 pandemic,...
Fda relaxing food labels. FDA rolls back ingredient and calorie labeling regulations, citing ... CBA applauded FDA's guidance, and food policy experts agreed that they were practical during the public health emergency. "Changing the ingredients used in food manufacturing can be a simpler and quicker process than creating new labels for the product packaging," said John Bovay, assistant professor of agricultural and applied economics at Virginia Tech. "Packaging and labeling is ... Myrbetriq Oral: Uses, Side Effects, Interactions, Pictures ... - WebMD Find patient medical information for Myrbetriq oral on WebMD including its uses, side effects and safety, interactions, pictures, warnings and user ratings. FDA Relaxes Food-Labeling Rules During COVID-19 Crisis John Fuson, a Crowell & Moring LLP partner and former associate chief counsel at the FDA, noted in a March 27 coronavirus comment that the relaxed nutrition labeling rule nevertheless calls for... Amazon.com: Magnilife Relaxing Leg Cream : Health & Household Statements regarding dietary supplements have not been evaluated by the FDA and are not intended to diagnose, treat, cure, or prevent any disease or health condition. Customer reviews. 4.3 out of 5 stars. 4.3 out of 5. 1,540 global ratings. 5 star 65% 4 star 15% 3 star 10% 2 star 5% 1 star 5% How customer reviews and ratings work View Image Gallery Amazon Customer. 3.0 …
Have a Food Allergy? What to Know About the New Relaxed Labeling Guidelines The FDA recently announced that it's relaxing some food labeling requirements because of the COVID-19 pandemic. With supply chains disrupted, they want to make it easier for manufacturers who can't... FDA Provides Flexibility to the Food Industry During COVID-19 To make this possible, late last month we issued new guidance indicating that the FDA, under certain conditions, will provide flexibility for restaurants and food manufacturers to sell packaged... FDA Relaxed Food Label Rules & Those With Allergies - Consumer Reports The FDA states that the ingredient being substituted into a food cannot be one of the top eight food allergens without disclosing it to consumers. (In addition to peanuts and eggs, the other main... FDA temporarily relaxes food-labeling guidelines to help food industry ... The FDA says it temporarily relaxed food-labeling guidelines to help the food industry deal with pandemic-related shortages of some ingredients. Now, food manufacturers can swap out some...
FDA Food Labeling Changes During COVID-19: Here's What ... - Parents FDA Food Labeling Changes During COVID-19: Here's What Parents Need to Know The FDA is relaxing some food labeling requirements in light of the pandemic. But these changes could be risky for those... How to Maintain Normal Blood Sugar - Dr. Axe 20.06.2017 · If you are one of the millions of people who has prediabetes, diabetes, metabolic syndrome or any other form of “insulin resistance,” maintaining normal blood sugar levels can be challenging. Over the past several decades, these chronic disorders have swept through the U.S. and many other nations, reaching epidemic proportions and causing serious, but often … FDA Provides Temporary Flexibility for Food Labeling | News ADVERTISEMENT 05/27/2020 FDA Provides Temporary Flexibility for Food Labeling The FDA has issued another guidance document to provide temporary flexibility with food labeling requirements to manufacturers and vending machine operators. The goal is to minimize the impact of supply chain disruptions amid the COVID-19 pandemic. Amazon.com: HoMedics White Noise Sound Machine | Portable ... About this item . 6 Soothing Sounds: Choose from 6 digitally recorded relaxing nature Sounds: White Noise, Thunder, Ocean, Rain, Summer Night, and Brook; The Soundspa's sounds are designed to mimic the natural environment in order to provide the most relaxing experience possible
Food allergy advocates worry about labeling changes - Detroit Free Press The Food and Drug Administration new relaxed food labeling requirements has food allergy concerned. ... The agency said it is relaxing the rules to avoid disruptions in the food chain.
The FDA Deserves Credit for Easing Food Ingredient Labeling Rules in ... (JOHN ANGELILLO/UPI/Newscom) Earlier this week, the Food and Drug Administration (FDA) announced it was relaxing food ingredient labeling rules due to ingredient shortages associated with the...
FDA eases labeling rules to ensure consumer access to food The guidance documents temporarily relax certain food labeling rules that if rigorously enforced may result in bottlenecks in the supply chain and threaten consumer access to food. The coronavirus...
FDA Announces Temporary Food Labeling During COVID-19 Pandemic First, the FDA is providing flexibility for manufacturers to make minor formulation changes in certain circumstances without making conforming label changes, such as making a change to product...
Is This Really the Time? FDA Relaxes Labeling Requirements for ... A restaurant can reuse original labels or provide the required information on labels it creates or that are provided by the manufacturer. Second, for food establishments that are part of a chain with 20 or more locations and offering substantially the same menu items, the FDA is relaxing menu labeling requirements during the public health ...
Valsartan Oral: Uses, Side Effects, Interactions, Pictures ... - WebMD Find patient medical information for valsartan oral on WebMD including its uses, side effects and safety, interactions, pictures, warnings and user ratings.
FDA temporarily relaxing some nutrition labeling rules The FDA previously said it would do so for the first six months of the year following the Jan. 1, 2020, compliance date. The updated guidance was published March 26, titled "Guidance for industry:...
FDA and USDA Temporarily Relax Nutrition Facts Labeling Requirement for ... FDA does not intend to object to the sale of packaged food that lacks a Nutrition Facts label by food manufacturers, provided that the food does not have any nutrition claims and contains other required information on the label. USDA's Food Safety and Inspection Service (FSIS) will not object to the use of labels or require establishments to ...
FDA Temporarily Relaxing Egg Labeling Requirement tom April 7, 2020. (IARN) — The Food and Drug Administration (FDA) is relaxing egg labeling and packaging requirements to meet consumer demands. Generally, egg cartons must include a statement ...
Herbal Medicine - Herbal Medicine - NCBI Bookshelf 05.11.2010 · Dietary supplements do not need approval from the Food and Drug Administration (FDA) before they are marketed . Under DSHEA, herbal medicines, which are classified as dietary supplements, are presumed safe, and the FDA does not have the authority to require them to be approved for safety and efficacy before they enter the market, which is the case for drugs. This …
San Francisco Restaurant Reviews, Recipes, Wine & Spirits - SFGATE Find food and wine reviews and news on San Francisco restaurants, recipes, cooking, chefs, cocktails and bars — SFGate
Manufacturers Pledging to Label All Ingredient Changes Despite FDA ... As some of you may be aware the Food and Drug Administration has relaxed the ingredient labeling rules for all products. Be assured, we at BeReal Doughs pledge not to relax our exceptionally high safety standards. The ingredient list on our packaging will always accurately reflect the ingredients within.
2020 Food Allergy Alert: FDA Temporarily Decreases Food Labeling ... As a result, the Food and Drug Administration (FDA) rolled out a new temporary regulation for relaxing food labeling requirements: Guidance for Industry: Temporary Policy Regarding Nutrition Labeling of Certain Packaged Food During the COVID-19 Public Health Emergency. This guidance provides flexibility to food manufacturers, labeling ...
The FDA relaxes food label rules for fifth time during the pandemic ... The FDA relaxes food label rules for fifth time during the pandemic - The Washington Post This article was published more than 2 years ago Business FDA rolls back food rules for 5th time during...
FDA relaxes certain food labeling laws -- it could put those with food ... Last Friday night, the U.S. Food and Drug Administration (FDA) announced a change, relaxing certain food labeling laws. It's intended to help manufacturers keep products on store shelves, but it ...
Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,...
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and ...
PDF Food and Drug Administration (FDA) 5001 Campus Drive - AAFA Director, Center for Food Safety and Applied Nutrition . Food and Drug Administration (FDA) 5001 Campus Drive . College Park, MD 20740 . Re: FDA-2020-D-1139 Temporary Policy Regarding Certain Food Labeling Requirements During the COVID-19 Public Health Emergency: Minor Formulation Changes and Vending Machines Guidance for Industry . Dear Dr. Mayne,
The FDA Has Relaxed Labeling Requirements Under COVID-19. What it Means ... In addition to the eight major food allergens defined at section 201 (qq) of the FD&C Act, several other foods (such as sesame, celery, lupin, buckwheat, molluscan shellfish, and mustard) are recognized as priority allergens in other parts of the world, including Canada, European countries, and Japan.
Good Manufacturing Practices for the 21st Century for Food … In an FDA survey of 85 small, medium, and large food plants, FDA and state inspectors found that only half of the firms were cross-checking ingredients on the labels with ingredients used in ...
FDA eases nutrition labeling rules to help processors sell restaurant food The FDA is relaxing nutrition labeling requirements during the COVID-19 pandemic to allow manufacturers to sell some packaged food that restaurants aren't buying to retailers experiencing surging demand. Restaurants, slow on business from closures or in-person dining bans, also can sell packaged ingredients that don't have Nutrition Facts labels required for retail.
FDA Provides Temporary Flexibility Regarding Nutrition Labeling of ... FDA Provides Temporary Flexibility Regarding Nutrition Labeling of Certain Packaged Food in Response to the COVID-19 Pandemic Constituent Update March 26, 2020 As a result of the COVID-19 pandemic,...
Amazon.com: Medisential Enema Kit - Suitable for Coffee, Water … Statements regarding dietary supplements have not been evaluated by the FDA and are not intended to diagnose, treat, cure, or prevent any disease or health condition. Statements regarding dietary supplements have not been evaluated by the FDA and are not intended to diagnose, treat, cure, or prevent any disease or health condition. Customer reviews. 4.6 out of 5 stars. 4.6 out …
Food Allergen Labeling—What Happens Next? - Celiac.com However, Covid-19 has changed that. Responding to calls from food manufacturers facing supply chain problems in the wake of the Covid-19 pandemic, the Food and Drug Administration (FDA) is relaxing some labeling requirements for certain ingredients in food in the U.S.































Post a Comment for "40 fda relaxing food labels"